Answer:

Step-by-step explanation:
Given the moles, we are asked to find the mass of a sample.
We know that the molar mass of methanol is 32.0 grams per mole. We can use this number as a fraction or ratio.
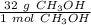
Multiply by the given number of moles, which is 2.0
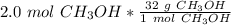
The moles of methanol will cancel each other out.
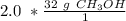
The denominator of 1 can be ignored.
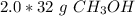
Multiply.
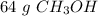
There are 64 grams of methanol in the sample.