Answer:
Half life period = 43.11 days
Explanation:
A sample substance has been taken for the use of drug research.
Weight of the substance taken = 0.25 gram
We have to use the formula of exponential decay to find the half life period of the substance.
Formula for the decay is
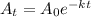
Where
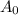 is the weight of the substance taken initially
is the weight of the substance taken initially
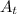 is the quantity remained after t time
is the quantity remained after t time
and t = time
Now we have to find the half life life period
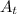 =
=
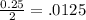
and
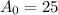
By putting these values in the formula
0.125 = 25
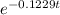
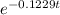 =
=
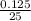
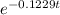 = 0.005
= 0.005
Now we take natural log on both the sides of the equation
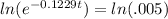
-0.1229t(lne) = -5.2983
0.1229t = 5.2983
t =
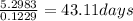 ≈ 43.10 days
≈ 43.10 days
Therefore, half life period of the substance is 43.10 days