Hydrolysis of
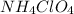 is given as:
is given as:
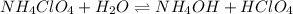
Here,
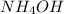 is a weak base and
is a weak base and
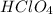 is a strong acid. Thus, solution is more acidic
is a strong acid. Thus, solution is more acidic
Hydrolysis of
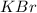 is given as:
is given as:
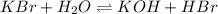
Here,
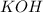 is a strong base and
is a strong base and
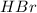 is a strong acid.Thus, solution is neutral.
is a strong acid.Thus, solution is neutral.
Hydrolysis of
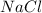 is given as:
is given as:
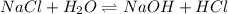
Here,
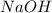 is a strong base and
is a strong base and
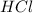 is a strong acid.Thus, solution is neutral.
is a strong acid.Thus, solution is neutral.
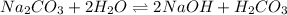
Here,
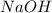 is a strong base and
is a strong base and
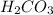 is a weak acid.Thus, solution is basic.
is a weak acid.Thus, solution is basic.
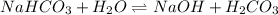
Here,
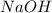 is a strong base and
is a strong base and
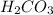 is a weak acid.Thus, solution is basic.
is a weak acid.Thus, solution is basic.
Hence, an aqueous solution of
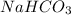 and
and
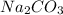 will produce a basic solution.
will produce a basic solution.