Answer: Option (c) is the correct answer.
Step-by-step explanation:
Entropy means the degree of randomness present in a substance or within the reactants in a chemical reaction.
Change in entropy is represented by
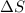 . More is the degree of randomness present more positive will be the value of
. More is the degree of randomness present more positive will be the value of
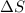 . Similarly, less is the degree of randomness present within a substance lesser will be the value of
. Similarly, less is the degree of randomness present within a substance lesser will be the value of
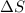 .
.
(a)
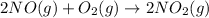
Here, 3 moles of reactants are giving 2 moles of product. Hence, entropy is decreasing so, the value of
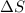 is negative.
is negative.
(b)
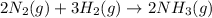
Here, 5 moles of reactants are giving 2 moles of product. Hence, entropy is decreasing so, the value of
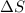 is negative.
is negative.
(c)
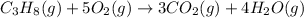
Here, 6 moles of reactants are giving 7 moles of product. Hence, entropy is increasing so, the value of
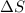 is positive.
is positive.
(d)
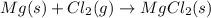
Here, 2 moles of reactants are giving 1 mole of product. Hence, entropy is decreasing so, the value of
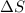 is negative.
is negative.
(e)
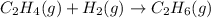
Here, 2 moles of reactants are giving 1 mole of product. Hence, entropy is decreasing so, the value of
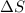 is negative.
is negative.
Thus, we can conclude that
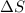 is positive for the reaction
is positive for the reaction
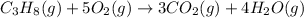 .
.