Step-by-step explanation:
Longest wavelengths of light that can cleave the bonds in elemental nitrogen
Energy to cleave 1 mol N-N bond = 945 kJ/mol = 945000 J/mol
1 mol=

Energy to break 1 N-N bond =
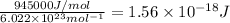
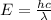
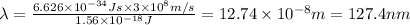
Longest wavelengths of light that can cleave the bonds in elemental nitrogen is 127.4 nm.
Similarly
For oxygen:
Energy to cleave 1 mol O-O bond = 498 kJ/mol = 498000 J/mol
Energy to break 1 O-O bond =
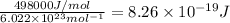
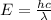
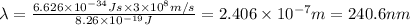
Longest wavelengths of light that can cleave the bonds in elemental oxygen is 240.6 nm.
For fluorine
Energy to cleave 1 mol F-Fbond = 159 kJ/mol = 159000 J/mol
Energy to break 1 F-F bond =
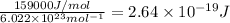
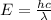
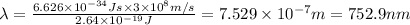
Longest wavelengths of light that can cleave the bonds in elemental fluorine is 752.9 nm.