Option B:
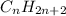
Alkane is defined as series of hydrocarbons which are saturated (all the carbon-carbon bonds are single) for example: methane, ethane, propane etc.
In alkane, carbon has 4 valence electrons so, it can make single bonds with 4 hydrogen atoms.
In the series, the first alkane is methane, it has 1 carbon atom thus, 4 hydrogen atoms are attached to it and molecular formula becomes
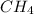 . Similarly, the second alkane in the series is ethane, it has 2 carbon atoms, both attached with single bond. The remaining three electrons in each carbon are shared with 3 hydrogen atoms each thus, it has total of 6 hydrogen atoms. Molecular formula of ethane will be
. Similarly, the second alkane in the series is ethane, it has 2 carbon atoms, both attached with single bond. The remaining three electrons in each carbon are shared with 3 hydrogen atoms each thus, it has total of 6 hydrogen atoms. Molecular formula of ethane will be
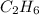 .
.
Now, for general formula, let the number of carbon atoms be n thus, number of hydrogen atoms will be
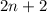
Therefore, general formula of straight chain alkane is
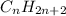 .
.