Answer : There is no change takes place in the oxidation number of Cl.
Explanation :
The given chemical reaction is,
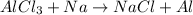
This reaction is an unbalanced reaction because the chlorine atoms are not balanced.
In order to balance the chemical reaction, the coefficient 3 is put before the Na and NaCl.
The balanced chemical reaction will be,
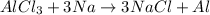
Now we have to calculate the oxidation number of all the elements.
In
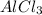 , the oxidation number of Al and Cl are, (+3) and (-1) respectively.
, the oxidation number of Al and Cl are, (+3) and (-1) respectively.
The oxidation number Na and Al are, zero (0)
In
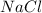 , the oxidation number of Na and Cl are, (+1) and (-1) respectively.
, the oxidation number of Na and Cl are, (+1) and (-1) respectively.
From this we conclude that the oxidation number of Cl changes from (-1) to (-1) that means remains same.
Therefore, there is no change takes place in the oxidation number of Cl.