Given: Flourine 23 has a half life of 2.2 seconds. Amount of Flourine 23 present at the beginning is 400 gm.
To find: The amount of Flourine 23 left after a duration of 11 seconds.
Solution:
The number of half-lives that 400 gm of Flourine 23 has to go through can be found by the following way,
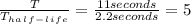
So the 400 gm of Flourine 23 will undergo 5 half lives and with each half-life the amount gets divided by 2 or reduces by half.
400 gm -> 200 gm -> 100 gm -> 50 gm -> 25 gm -> 12.5 gm
∴ 12.5 gm of Flourine 23 will be left after 11 seconds.