Hydrate of magnesium sulphate undergoes dehydration upon action of heat. This process can be chemically represent as follows:
MgSO4.XH2O → MgSO4 + XH20
(5.018 g) (2.449 g) (2.569 g)
Number of moles of H2O evolved =
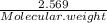
=
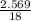
= 0.1427
Number of moles of MgSO4 generated after decomposition,
=
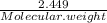
=
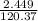
=0.0203
Now dividing both these numbers by the smallest
one to get the mole ratio that exists between MgSO4 and H20
For H2O,
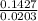 = 7.02 ~7
= 7.02 ~7
Thus, formula of hydrate is MgSO4.7H2O