Answer: The relationship between energy and frequency of a photon is given by E = h*f.
E is the energy, h is planck constant and f is the frequency and f= c/λ.
But i want wavelength, so i write this equation as E= h*c/λ.
now
c=3.8
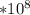
E =4.38*
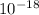
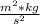
where i replaced joules for
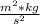
h = 6.62607004 *
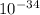
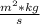
then λ = h*c/E =
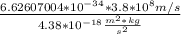 = 5.7*
= 5.7*
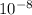 m
m
But you want the solution in angstroms, so 1 meter is
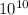 angstroms
angstroms
so λ = 5.7*
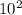 = 570 angstrom
= 570 angstrom