According to
Graham's Law of Diffusion,"the rates of diffusion of two gases are inversely proportional to the square root of their Molar masses or Densities at the same pressure and temperature".
r₁ / r₂ =
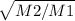
Where,
r₁ = Rate of Helium
r₂ = Rate of Oxygen
M₂ = Molar mass of Oxygen = 32 g/mol
M₁ = Molar mass of Fluorine = 4 g/mol
Putting values,
r₁ / r₂ =
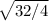
r₁ / r₂ =
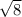
r₁ / r₂ =
2.82
Result:
Helium gas effuses
2.82 times faster than Oxygen gas.