STP stands for Standard Temp. and Pressure.
Standard Temp. = 273.15 K
Pressure = 1 atm
Now, ideal gas equation we know that PV = nRT
where, V = volume of gas = 0.475 l
R = universal gas constant = .082507 l atm mol-1 K-1
∴ n =
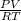
∴ n =
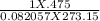
∴n = 0.02112 mol
Thus, number of moles of Ar present = 0.02112
But, number of mole =
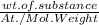
In present case, weight of Ar (g) = number of mole of Ar X Atomic Wt. of Ar
∴ weight of Ar = 40 X 0.02112 = 0.8477 g