Before addition of HCl,
conc. of CH3COOH = 0.450 M
conc. of CH3COONa = 0.550 M
After addition of 0.08 M HCl, following reaction occurs in system:
HCl + CH3COONa ↔ CH3COOH + NaCl
Thus, in reaction system conc. of CH3COOH will increase to 0.53 M (0.08M + 0.450M)
And, conc to CH3COONa will reduce to 0.47 M (0.550M - 0.08M)
Now, conc. of H+ ions = ka
![([acid])/([conjugated base])](https://img.qammunity.org/2019/formulas/chemistry/college/itm3rihudxsq9az18xj8fzt6x2mxb4cowy.png)
where ka = dissociation constant for acid = 10^-5 for Ch3COOH
∴ conc. of H+ ions =
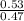
= 1.1277 x 10^-5
Now, pH = -log [H+] = -log (1.1227 x 10^-5) = 4.94