Answer:
The new volume of the sample will be 300 milliliteres
Step-by-step explanation:
We were given the following;
Initial Volume (V1) = 150ml
Initial Temperature (T1) = 300K
Final Temperature (T2) = 600K
Final Volume (V2) = ?
Pressure is held constant.
The law applicable in solving this question is that of Charle's law. This law states that the volume of a fixed mass of gas varies proportional to the temperature at constant pressure.
The formula is given as;
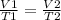
Solving for V2. we have;
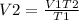
V2 = ( 150 * 600) / 300
V2 = 300 ml