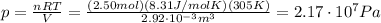I guess you meant "at
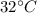
"
Solution:
we can solve the problem by using the ideal gas law, which states:
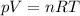
where
p is the gas pressure
V its volume
n the number of moles
R the gas constant
T the absolute temperature
Before using this equation, we have to convert the temperature in Kelvin:
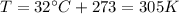
and the volume in
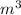
:
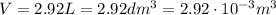
So now we can re-arrange the ideal gas equation to find the pressure exerted by the gas, p: