Answer:
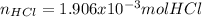
Step-by-step explanation:
Hello,
Titration is widely used to determine the neutralized moles of either an acid or base. In this case, the idea is to titrate (neutralize) hydrochloric acid with sodium hydroxide based on the following reaction:

Thus, one computes the neutralized moles of hydrochloric acid (equivalence of moles) as long as the mole ratio between the acid and the base is 1 to 1 and the moles of both of them must be equal for the neutralization to be successfully carried out as shown below:
Best regards.