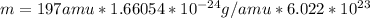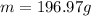Step-by-step explanation:
The mass number of an element is a weighted average of all the isotopes of that element.
If Au has only one isotope then the atomic weight of that isotope matches the mass number.
Au-197 ==> mass number 197
Mass of one atom=mass number 197 amu
Mass of one mol of atoms: