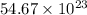Answer : The number of atoms of copper will be,
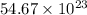
Solution :
As we know that 1 mole contains
 number of atoms
number of atoms
And,
At STP, 1 mole contains 22.4 liter volume of gas
First we have to calculate the moles of copper.
The balanced chemical reaction will be,
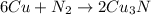
From the balanced chemical reaction we conclude that, 1 mole of nitrogen gas react with 6 moles of copper.
As, 22.4 L volume contains 6 moles of copper
So, 33.9 L volume contains
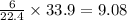 moles of copper
moles of copper
Now we have to calculate the number of atoms of copper.
As, 1 mole of copper contains
 number of copper atoms
number of copper atoms
So, 9.08 moles of copper contains
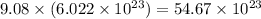 number of copper atoms
number of copper atoms
Therefore, the number of atoms of copper will be,