Answer: 101.367 grams of chlorine gas.
Step-by-step explanation:
Pressure of the chlorine gas =P = 2.85 atm
Volume occupied by the gas = 12.5 L
Temperature of the gas = 27°C= 300 K (0°C = 273 K)
Number of moles of chlorine gas = n
PV = nRT
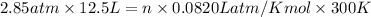
n = 1.4481 moles
Mass of the chlorine gas : 1.4481 moles × 70 g/mol = 101.367 g
101.367 grams of chlorine gas.