Answer : Oxidation reaction produces oxygen and water molecule during electrolysis of water.
Step-by-step explanation:
Electrolysis : It is a type of process in which the chemical decomposition produced by passing an electric current through the solution that containing the ions.
Electrolysis of water : It is basically the decomposition of water into oxygen and hydrogen gas through a process of electric current.
Oxidation reaction is defined as the reaction in which a substance looses its electron to attain stability.
The chemical reactions during electrolysis of water are:
At cathode:
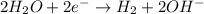
At anode:
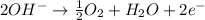
Overall reaction:

Oxidation reaction occurs at anode.
Hence, oxidation reaction produces oxygen and water molecule during electrolysis of water.