Step-by-step explanation:
It is given that,
Mass of the unknown substance, m = 13.5 gm
Volume of the substance,
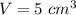
We need to tell the unknown sample is made up of. It can be indicated using the density of the sample. It can be calculated as :
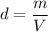
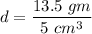
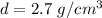
So, the density of the sample is
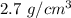 . The unknown sample is aluminium. Hence, this is the required solution.
. The unknown sample is aluminium. Hence, this is the required solution.