Answer: Option (b) is the correct answer.
Step-by-step explanation:
Freezing point is defined as the point at which liquid state of a substance changes into solid state.
So, in the given table it is shown that at temperature
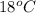 the substance is changing into solid state. Whereas at
the substance is changing into solid state. Whereas at
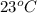 the substance is in liquid state.
the substance is in liquid state.
Hence,
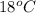 will be the freezing point of the substance.
will be the freezing point of the substance.
Thus, we can conclude that the statement its freezing point is
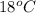 , is correct about the unknown substance.
, is correct about the unknown substance.