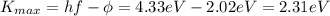In the photoelectric effect, the energy of the incoming photon is used partially to extract the photoelectron from the metal (work function) and the rest is converted into kinetic energy of the photoelectrons:
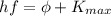
(1)
where
h is the Planck constant
f is the photon frequency
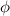
is the work function
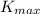
is the maximum kinetic energy of the photoelectrons
In the first part of the problem, we have light with wavelength
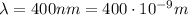
, so the photons have frequency
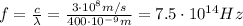
where c is the speed of light; and therefore the energy of the photons is
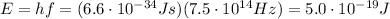
Converted into electronvolts (
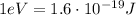
):
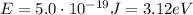
The maximum kinetic energy of the photoelectrons in this case is

, so we can find the work function of the metal by re-arranging eq. (1):

In the second part of the problem, we have light with wavelength
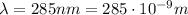
, which corresponds to a frequency of
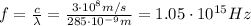
And so the photons have energy
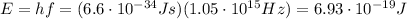
which corresponds to
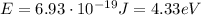
And if we use again eq.(1), we can now find the new maximum kinetic energy of the photoelectrons: