we can use the combined gas law equation to solve for the initial volume
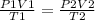
parameters for the first instance are on the left side and parameters for the second instance are on the right side of the equation
substituting the values in the equation
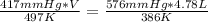
V = 8.50 L
the initial volume is 8.50 L