Answer : The volume of ammonia gas at STP is, 67.2 liters
Explanation : Given,
Mass of ammonia = 51.0 g
Molar mass of ammonia = 17 g/mole
As we know that,
At STP, 1 mole of gas contains 22.4 L volume of gas.
First we have to calculate the moles of ammonia gas.
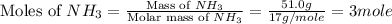
Now we have to calculate the volume of ammonia gas at STP.
As, 1 mole of ammonia gas contains 22.4 L volume of ammonia gas
So, 3 moles of ammonia gas contains
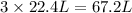 volume of ammonia gas
volume of ammonia gas
Therefore, the volume of ammonia gas at STP is, 67.2 liters