Answer:
The values of Kb and Kw
Step-by-step explanation:
The conjugated acid would produced when we have the reaction:

The equilibrium of this reaction is rule the Kb expression. If we want to solve Ka we have to use the equation:
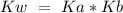
Solving for Ka:
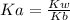
Therefore we need to know Kw and Kb.