The common names are given to compounds following common rules. They are named on the basis of there constituent ions.
(1)
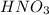 contains hydrogen ion and nitrate ion. It can give its hydrogen ion in the aqueous solution thus, it is an acid. Therefore, it is commonly known as nitric acid.
contains hydrogen ion and nitrate ion. It can give its hydrogen ion in the aqueous solution thus, it is an acid. Therefore, it is commonly known as nitric acid.
(2) HCl contains hydrogen ion and chloride ion. It can give its hydrogen ion in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as hydrochloric acid.
(3)
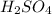 contains 2 hydrogen ions and 1 sulfate ion. It can easily give its hydrogen ions in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as sulfuric acid.
contains 2 hydrogen ions and 1 sulfate ion. It can easily give its hydrogen ions in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as sulfuric acid.
(4) HF contains hydrogen ion and fluoride ion. It can give its hydrogen ion in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as hydrofluoric acid.
(5)
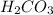 contains 2 hydrogen ions and 1 carbonate ion. It can give its hydrogen ion in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as carbonic acid.
contains 2 hydrogen ions and 1 carbonate ion. It can give its hydrogen ion in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as carbonic acid.
(6)
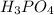 contains 3 hydrogen ions and 1 phosphate ion. It can give its hydrogen ion in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as phosphoric acid.
contains 3 hydrogen ions and 1 phosphate ion. It can give its hydrogen ion in the aqueous solution thus, it is also an acid. Therefore, it is commonly known as phosphoric acid.
Therefore, the acids with their common names are as follows:
(1)
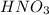 : Nitric acid
: Nitric acid
(2) HCl: Hydrochloric acid
(3)
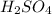 : Sulfuric acid
: Sulfuric acid
(4) HF: Hydrofluoric acid
(5)
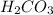 : Carbonic acid
: Carbonic acid
(6)
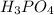 : Phosphoric acid
: Phosphoric acid