Answer: Chemical bonds in the water molecule are broken.
Step-by-step explanation:
A physical change is defined as a change in which there is alteration in shape, size etc. No new substance gets formed in these reactions.
Example: freezing of water
A chemical change is defined as a change in which a change in chemical composition takes place. A new substance is formed in these reactions.
Example: electrolysis of water
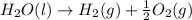
This is a decomposition reaction in which one reactant gives two or more than two products.
As the bonds between water are broken to form new bonds for formation of hydrogen and oxygen, the change is considered as chemical change.