Explanation:
Hey! Let's help you with that!
So, to start, I'm going to guide you through the first question and provide a detailed explanation on how to do this. There is a shortened explanation at the end if you don't want to have to read through this long explanation over and over again when solving the other questions but I do recommend you reading through the detailed explanation at least once or twice to get a good understanding.
To start, when balancing equations, your goal is to essentially make the number of elements on the left side equal the right side. Now what does that mean? Well, for this explanation, I'm going to be using a list to show it but to start, we want to split all of elements separately, so it would be as such:
Left Side Elements:

- Na (Sodium)
- P (Phosphorus)
- O (Oxygen)
- K (Potassium)
- H (Hydrogen
Right Side Elements:
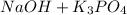
- Na (Sodium)
- P (Phosphorus)
- O (Oxygen)
- K (Potassium)
- H (Hydrogen
Now that we have all of them split up, we want to write down how many atoms there are of each element. To get how many atoms of an element, take a look at the subscript numbers after the element, anything without a subscript is considered to be just 1. So this would be:
Left Side Elements:
- Na (Sodium) = 3
- P (Phosphorus) = 1
- O (Oxygen) = 5 (4 from the first compound, one from the second)
- K (Potassium) = 1
- H (Hydrogen) = 1
Right Side Elements:
- Na (Sodium) = 1
- P (Phosphorus) = 1
- O (Oxygen) = 5 (4 from the first compound, one from the second)
- K (Potassium) = 3
- H (Hydrogen) = 1
Notice from what I've highlighted, these two elements have different amounts of atoms and the whole goal is to get these numbers equal to each other. So what do we do? We add a coefficient in front of the compound, in order to have left side equal right side.
Let's isolate two compounds
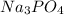 (from left side) and
(from left side) and
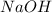 (from right side). Here, we have
(from right side). Here, we have
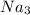 for the left side and just
for the left side and just
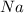 on the right. In order from them to equal each other, the
on the right. In order from them to equal each other, the
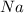 on the right needs to be multiplied by 3 to match the amount of
on the right needs to be multiplied by 3 to match the amount of
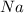 on the left but what does that do?
on the left but what does that do?
Now it becomes
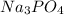 (left side) and
(left side) and
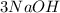 (right side). The coefficient is multiplied into all elements, so both left and right side
(right side). The coefficient is multiplied into all elements, so both left and right side
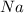 both equal to 3, but when putting a coefficient in front of a compound, it doesn't just multiply a single element, it multiplies the entire compound by 3 also, so hydrogen and oxygen are also going to be multiplied by 3.
both equal to 3, but when putting a coefficient in front of a compound, it doesn't just multiply a single element, it multiplies the entire compound by 3 also, so hydrogen and oxygen are also going to be multiplied by 3.
Essentially, you are just adding coefficients until every number of atoms on the left side equals the right side. To shorten this explanation, I will just show you how I did this question and my process. (the = sign is the same as the right arrow sign)
Answer:
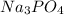 + 3
+ 3
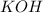 = 3
= 3
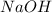 +
+
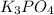
Left Side Elements:
- Na (Sodium) = 3
- P (Phosphorus) = 1
- O (Oxygen) = 7 (4 from
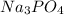 and 3 from
and 3 from
 )
) - K (Potassium) = 3
- H (Hydrogen) = 3
Right Side Elements:
- Na (Sodium) = 3
- P (Phosphorus) = 1
- O (Oxygen) = 7 (3 from
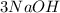 and 4 from
and 4 from
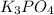 )
) - K (Potassium) = 3
- H (Hydrogen) = 3
As you can see all elements on the left side have the same set of atoms as the right side. Therefore, it is balanced. If there are any more questions, feel free to let me know! Best of luck!
Shortened Explanation:
- Write out each element and separate them from left and right side.
- Write down how many atoms of each element on each side.
- Add coefficients so that the number of atoms of each element is the same on both sides.
- Check by calculating how many atoms of each element are on both sides, if they are all equal, they are balanced.