Answer:
1. 412atm
2. 300atm
Step-by-step explanation:
Hello,
1. In this case, Van der Waals equation is suitable to model the non-ideal behavior of hydrogen at the specified conditions:
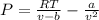
At the specified conditions, we compute
 ,
,
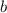 and
and
 as shown below:
as shown below:
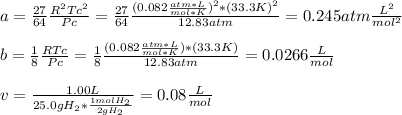
Thus, the pressure turns out into:
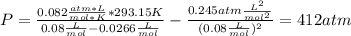
2. In this section, the ideal gas equation is directly applied as shown below:
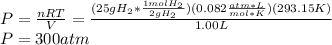
Best regards.