Answer : The correct answer is, Tetrahedral.
Explanation :
VSEPR Theory : It predict the geometry of the molecule from the number of electrons pair that surround the central atom.
Formula used :
Number of electrons =
![(1)/(2)[V+N-C+A]](https://img.qammunity.org/2019/formulas/chemistry/college/behunmw9o38v8waaz7fjxke5kwwn5ol1qv.png)
where,
V = number of valence electrons present in central atom
N = number of neighboring atoms
C = charge of cation
A = charge of anion
In the given molecule, S is the central atom and F is the neighboring atom.
Sulfur has 6 valence electrons.
Number of electrons =
![(1)/(2)[6+2-0+0]=4](https://img.qammunity.org/2019/formulas/chemistry/high-school/760zysu185d4w4klnwhbsvxolukbekbfll.png)
The number of electrons is 4 that means the hybridization will be
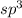 and the electron domain geometry will be tetrahedral.
and the electron domain geometry will be tetrahedral.
The electron domain geometry is shown below.