The ionization reaction of water is as follows:
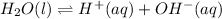
The expression for ionization constant is written as follows:
![K=([Product])/([Reactant])](https://img.qammunity.org/2019/formulas/chemistry/high-school/umczvx7kndjje23qr9ccigza46mvgzrmqc.png)
In the expression, reactant and product only in gaseous and aqueous form are written. Reactant and product in solid and liquid state are not written in the expression for ionization constant. Thus,
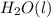 will not be there in the expression.
will not be there in the expression.
Therefore, expression for ionization constant of water will be:
![K_(W)=[H^(+)][OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/opcm8qwiolfpwaniemm9ui23dbubugh12q.png)
Here,
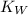 is ionization constant of water,
is ionization constant of water,
![[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/75vk4lm3dg3i0qv728qhnrrb5qib1loutc.png) is concentration of hydrogen ion and
is concentration of hydrogen ion and
![[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbzut40quqceef230a4l1arx1nzd8511gj.png) is concentration of hydroxide ion.
is concentration of hydroxide ion.