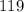Neutrons are the subatomic particles present in an atom having no charge i.e. neutral and mass of the neutron is approximately same as the mass of the proton.
Number of neutrons is equal to the subtraction of atomic mass and atomic number.
Number of neutrons = Atomic mass - atomic number
Atomic number of gold is 79 and atomic mass of gold is 198 amu.
Substitute the above value in formula, we get
Number of neutrons =

=
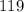
Thus, number of neutrons =