Answer: This ion has 13 protons, 14 neutrons and 10 electrons.
Step-by-step explanation:
An ion is formed when an atom looses or gains electron.
- When an atom looses electrons, it will form a positive ion known as cation.
- When an atom gains electrons, it will form a negative ion known as anion.
Atomic number is defined as the number of protons or electrons that are present in a neutral atom.
Atomic number = number of protons = number of electrons
Atomic mass is defined as the sum of number of protons and neutrons that are present in an atom.
Atomic mass = Number of protons + Number of neutrons
For the given representation of ion, which is:
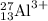
We are given:
Atomic number = Number of protons = 13
Number of electrons = Number of protons - charge = 13 - (+3) = 10
Mass number = 27
Number of neutrons = Atomic number - Mass number = 27 - 13 = 14
Hence, this ion has 13 protons, 14 neutrons and 10 electrons.