Answer:
Step-by-step explanation:
the reaction is
Zn(s) + 2MnO₂(s) + H₂O(l) ⟶ Zn(OH)₂(s) + Mn₂O₃(s)
This is the discharge reaction, where Zn is undergoing oxidation and Mn is undergoing reduction.
The anode reaction is:
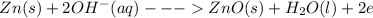
The cathode reaction is:

Thus here MnO₂ is undergoing reduction (and the element undergoing reduction is Mn).