Answer: Chemical energy, product Z have is 318.1 J.
Explanation: Chemical energy is the energy stored between the bond of the compound.
For a given chemical reaction:
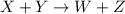
We are given,
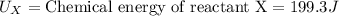
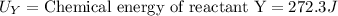
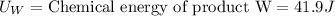
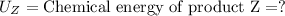
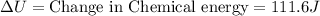
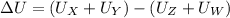
Putting the values in above equation, we get
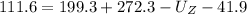
Rearranging the terms, we get
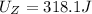
Chemical energy product Z have is 318.1 J.