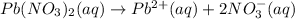Answer : The balanced chemical reaction will be,
Explanation :
Balanced chemical reaction : It is defined as the chemical reaction in which the number of individual atoms of an element in reactant side always be equal to the number of individual atoms of an element in product side.
The given balanced reaction will be,
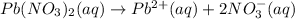
By the stoichiometry we can say that, 1 mole of
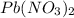 dissociates into 1 mole of
dissociates into 1 mole of
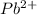 ion and 2 moles of
ion and 2 moles of
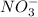 ions.
ions.
Hence, the balanced chemical reaction will be,