Answer:- 1:1
Explanations:- The given balanced equation is:
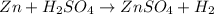
Looking at the balanced equations, the mol ratios could easily be figured abut as the mol ratio is the ratio of their coefficients. In this equation, the coefficients for all of them is 1. It means 1 mol of zinc is reacting with 1 mol of sulfuric acid to form 1 mol of zinc sulfate and 1 mol of hydrogen gas.
So, the mol ratio of zinc to zinc sulfate is 1:1.