Answer:
The correct answer is option (a).
Step-by-step explanation:
The molecular formula of lithium carbonate is
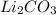
Percentage of lithium in lithium carbonate = 18.8 %
Molar mass of the lithium carbonate = M
Atomic mass of lithium = 7 g/mol
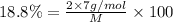
M = 74.46 g\mol
Mass of pure lithium carbonate is twice the mass of first sample:
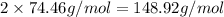
Percentage of lithium in pure lithium carbonate sample:
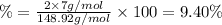
The correct answer is option (a).