a) initial volume
We can calculate the initial volume of the gas by using the ideal gas law:
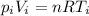
where
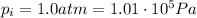
is the initial pressure of the gas
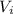
is the initial volume of the gas
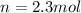
is the number of moles
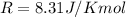
is the gas constant
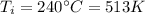
is the initial temperature of the gas
By re-arranging this equation, we can find
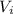
:
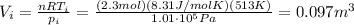
2) Now the gas cools down to a temperature of
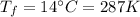
while the pressure is kept constant:
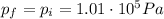
, so we can use again the ideal gas law to find the new volume of the gas
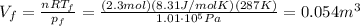
3) In a process at constant pressure, the work done by the gas is equal to the product between the pressure and the difference of volume:
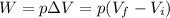
by using the data we found at point 1) and 2), we find
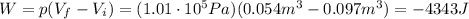
where the negative sign means the work is done by the surrounding on the gas.