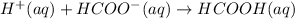Step-by-step explanation:
An ionic equation is an equation in which the given reactant species or electrolytes are written in the form of dissociated ions in the presence of a solution.
For example,
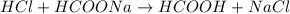
The ionic equation for the same will be as follows.
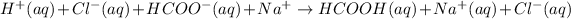
Therefore, cancelling common ions from, that is,
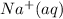 and
and
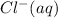 from both sides we get the net ionic equation as follows.
from both sides we get the net ionic equation as follows.