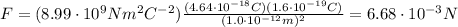The charge of the copper nucleus is 29 times the charge of one proton:
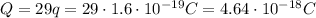
the charge of the electron is
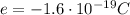
and their separation is
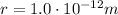
The magnitude of the electrostatic force between them is given by:
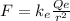
where
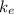
is the Coulomb's constant. If we substitute the numbers, we find (we can ignore the negative sign of the electron charge, since we are interested only in the magnitude of the force)