The momentum of a photon is given by the following relationship:
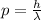
where
h is the Planck constant
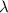
is the photon wavelength
For the photon in our problem,
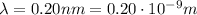
, so its momentum is
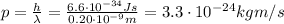
The electron must have the same momentum of this photon, and its momentum is given by (in the non-relativistic approximation)
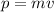
where
m is the electron mass
v is its speed
Re-arranging this formula, we can calculate the electron speed:
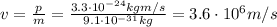
And this velocity is quite small compared to the speed of light (
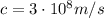
), so the non-relativistic approximation that we used for the electron's momentum is valid.