the balanced reaction between NaOH and HCl is as follows
NaOH + HCl ----> NaCl + H₂O
stoichiometry of NaOH to HCl is 1:1
molar mass of NaOH is 40 g/mol
number of moles =
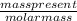
therefore number of NaOH moles - 4.00 g / 40 g/mol = 0.100 mol
since molar ratio of HCl to NaOH is 1:1
number of HCl moles required = 0.100 mol
Molarity of HCl is 1.0 M
this means that a volume of 1 L contains 1.0 mol
therefore volume containing 0.100 mol - 0.100 mol / 1.0 mol/L = 0.10 L
volume of HCl required = 100 mL