Answer: A)
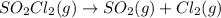
Explanation: Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
A)
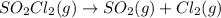
1 mole of gaseous reactant is converting to 2 gaseous products, thus the entropy increases.
B)
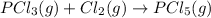
2 moles of gaseous reactants are converting to 1 gaseous products, thus the entropy decreases.
C)
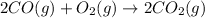
3 moles of gaseous reactants are converting to 1 gaseous product, thus the entropy decreases.
D.
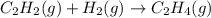
2 moles of gaseous reactants are converting to 1 gaseous product, thus the entropy decreases.