Answer:
Step-by-step explanation:
Gay-Lussac's Law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas, when the volume is kept constant.
P₁=84kPa
P₂=?kPa
The temperatures have first been converted to Kelvin.
T1=30°C
Converting to kelvin; 30+273= 303K
T2=240°C
Converting to kelvin; 240+273 = 513K
Step 1: Use Gay-Lussac's Law to solve for the unknown pressure (P₂).
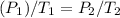
Step 2: Solve.
First, rearrange the equation algebraically to solve for P₂.
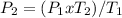
Now substitute the known quantities into the equation and solve.
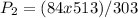
P₂ = 142kPa