Answer: D)supersaturated
Step-by-step explanation: Solubility is defined as the amount of solute in grams which can dissolve in 100 g of the liquid to form a saturated solution at that particular temperature.
At
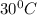 , the solubility of
, the solubility of
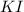 is 153g/100 ml.
is 153g/100 ml.
Thus if 180 grams is dissolved, it contains more amount of solute than it can hold at that that temperature, and thus is supersaturated solution.
A saturated solution is a solution containing the maximum concentration of a solute dissolved in the solvent. The additional solute does not dissolve in a saturated solution.
An unsaturated solution is solution in which the solute concentration is lower than its equilibrium solubility.
A supersaturated solution is one that has more solute than it can hold at a certain temperature.