the mass of a carbon atom is 1.994 x 10⁻²³ g
the mass of the carbon sample is 12.01 g
to find the number of Carbon atoms we have to divide the mass of sample by mass of a carbon atom.
number of C atoms =
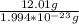
therefore number of atoms = 6.023 X 10²³ atoms of carbon