Answer:
The
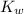 value of water at 50.0° C is
value of water at 50.0° C is
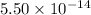 .
.
Step-by-step explanation:
The pH of water at 50.0° C = 6.630
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
![6.630=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/college/gv6t20v30wzvx02xeva7u9h4vslym3ey83.png)
![[H^+]=2.344* 10^(-7)M](https://img.qammunity.org/2019/formulas/chemistry/college/cjmjsq6jg0m57amwnpbq9xupsnpl60s3te.png)
Since, water is a neutral compound wuith equal number of hydrogen ions and hydroxide ions.
![[OH^-]=[H^+]=2.344* 10^(-7)M](https://img.qammunity.org/2019/formulas/chemistry/college/8zsb8rpxueqenz17nc60wmhjsn0at888ja.png)
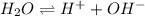
The expression for an ionic product of water is given as:
![K_w=[H^+][OH^-]](https://img.qammunity.org/2019/formulas/chemistry/college/i9bubc7hlt004yxe0p864o2430bgzoojnh.png)
Substituting the values:
![K_w=[2.344* 10^(-7)M][2.344* 10^(-7)M]=5.49* 10^(-14)\approx 5.50* 10^(-14)](https://img.qammunity.org/2019/formulas/chemistry/college/ao7n4bjetzbabfh9q8qhg9uv58jrxal76w.png)
The
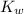 value of water at 50.0° C is
value of water at 50.0° C is
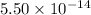 .
.