we can use the combined gas law equation to find volume of gas
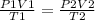
P - pressure
V - volume
T - temperature
parameters at STP conditions are on the left side and parameters for the second instance are on the right side of the equation
standard temperature is 273 K and standard pressure is 10⁵ Pa
substituting the values in the equation
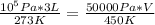
V = 9.89 L
new volume is 9.89 L
answer is
B) 9.89 L